Activation Energy Change With Temperature . a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. The only way to explain. The activation energy can also be calculated. in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. the activation energy can be graphically determined by manipulating the arrhenius equation.
from www.animalia-life.club
thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. The activation energy can also be calculated. in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy can be graphically determined by manipulating the arrhenius equation. The only way to explain.
Activation Energy And Temperature
Activation Energy Change With Temperature the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. The activation energy can also be calculated. The only way to explain. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy can be graphically determined by manipulating the arrhenius equation. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the.
From www.animalia-life.club
Activation Energy And Temperature Activation Energy Change With Temperature in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. the activation energy can be graphically determined by manipulating the arrhenius equation. thus, the proportion. Activation Energy Change With Temperature.
From www.vrogue.co
Map Of Activation Energy Of High Temperature Deformat vrogue.co Activation Energy Change With Temperature in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. The only way to explain. thus, the proportion of collisions that can overcome the activation energy. Activation Energy Change With Temperature.
From thechemistrynotes.com
Activation Energy Definition, Unit, Formula, Calculations Activation Energy Change With Temperature the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. The only way to explain. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. thus, the proportion of collisions that can overcome. Activation Energy Change With Temperature.
From schematiclistmotus123.z13.web.core.windows.net
Energy Diagram Of Exothermic Reaction Activation Energy Change With Temperature a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. the activation energy can be graphically determined by manipulating the arrhenius equation. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. the activation energy (ea e a), labeled. Activation Energy Change With Temperature.
From lessonfullbatholite.z21.web.core.windows.net
How To Read Energy Diagrams Chemistry Activation Energy Change With Temperature thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and. Activation Energy Change With Temperature.
From www.animalia-life.club
Activation Energy And Temperature Activation Energy Change With Temperature the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. The activation energy can also be calculated. in this section, we will use the collision model to. Activation Energy Change With Temperature.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Activation Energy Change With Temperature The only way to explain. the activation energy can be graphically determined by manipulating the arrhenius equation. the activation energy (ea e a), labeled δg‡ δ g ‡ in figure 2, is the energy difference between the reactants and the. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature.. Activation Energy Change With Temperature.
From www.animalia-life.club
Activation Energy And Temperature Activation Energy Change With Temperature in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. The activation energy can also be. Activation Energy Change With Temperature.
From circuitdiagramlows.z22.web.core.windows.net
What Is The Activation Energy On A Diagram Activation Energy Change With Temperature The only way to explain. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the. Activation Energy Change With Temperature.
From schematicdarkrose51r3.z14.web.core.windows.net
Energy Diagram Activation Energy Activation Energy Change With Temperature the activation energy can be graphically determined by manipulating the arrhenius equation. The activation energy can also be calculated. The only way to explain. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate. Activation Energy Change With Temperature.
From www.animalia-life.club
Activation Energy And Temperature Activation Energy Change With Temperature apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. The activation energy can also be calculated. a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. the activation energy can be graphically determined by manipulating the arrhenius equation. . Activation Energy Change With Temperature.
From masterconceptsinchemistry.com
What’s reaction rate? How temperature, concentration, and catalyst Activation Energy Change With Temperature the activation energy can be graphically determined by manipulating the arrhenius equation. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. the activation energy (ea e a), labeled. Activation Energy Change With Temperature.
From www.animalia-life.club
Activation Energy And Temperature Activation Energy Change With Temperature The activation energy can also be calculated. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy can be graphically determined by manipulating the arrhenius. Activation Energy Change With Temperature.
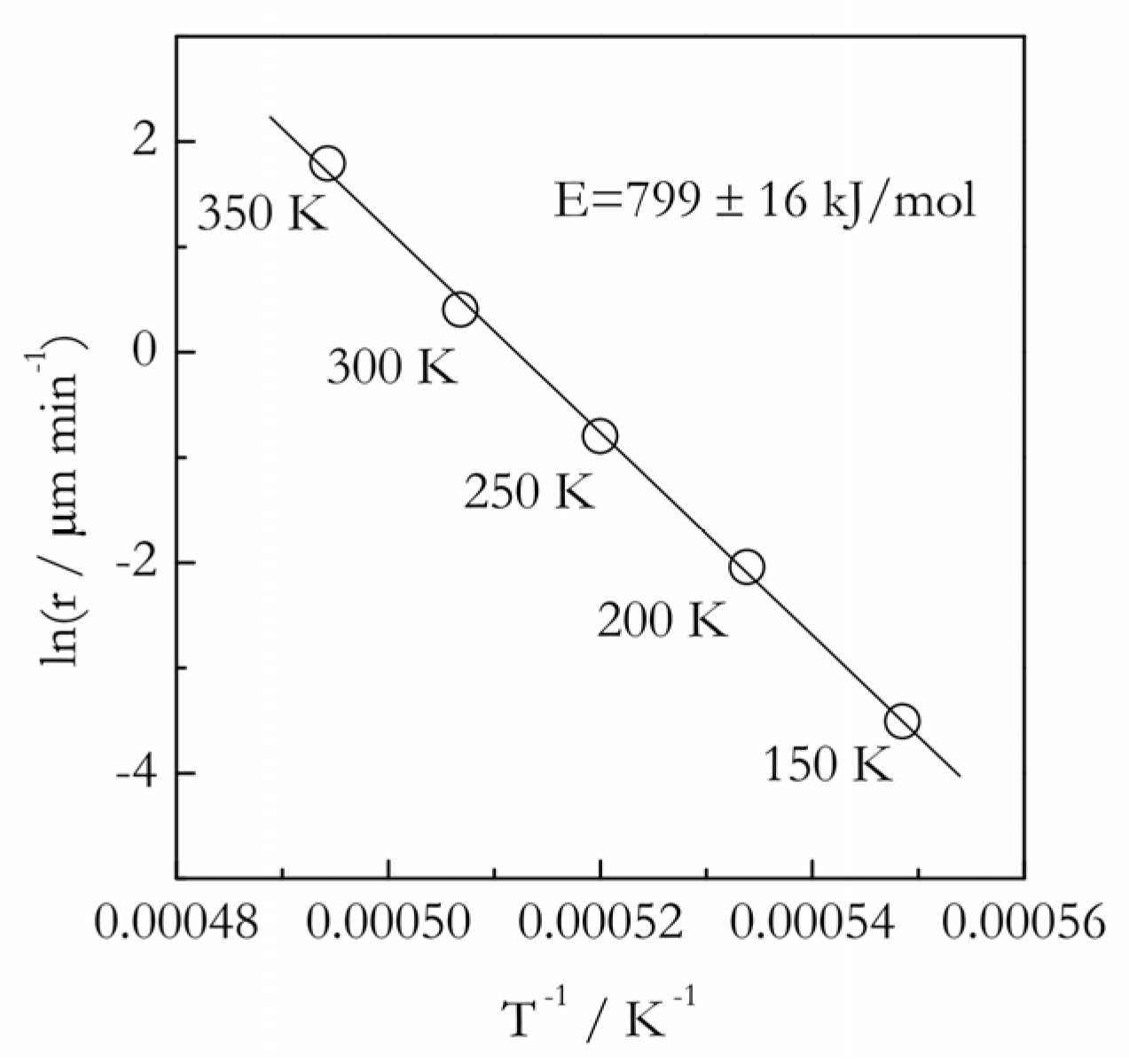
From www.researchgate.net
Temperature dependence of the activation energy in the temperature Activation Energy Change With Temperature in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. the activation energy can be graphically determined by manipulating the arrhenius equation. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. a higher temperature represents a correspondingly greater fraction of. Activation Energy Change With Temperature.
From www.slideserve.com
PPT Section 14.5 Activation Energy and Temperature PowerPoint Activation Energy Change With Temperature in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. The activation energy can also be calculated. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. the activation energy can be graphically determined by manipulating the arrhenius equation. the activation. Activation Energy Change With Temperature.
From www.animalia-life.club
Activation Energy And Temperature Activation Energy Change With Temperature the activation energy can be graphically determined by manipulating the arrhenius equation. The activation energy can also be calculated. thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics.. Activation Energy Change With Temperature.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation ID4450666 Activation Energy Change With Temperature thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. The activation energy can also be calculated. The only way to explain. in this section, we will use the. Activation Energy Change With Temperature.
From chemistry.stackexchange.com
Why on changing temperature of a reaction its activation Activation Energy Change With Temperature a higher temperature represents a correspondingly greater fraction of molecules possessing sufficient energy (rt) to overcome. apply the arrhenius equation to illustrate the effects of temperature and activation energy on reaction rate and recognize the reaction characteristics. in this section, we will use the collision model to analyze this relationship between temperature and reaction rates. The activation. Activation Energy Change With Temperature.